When you take two medications together - like amlodipine and benazepril for high blood pressure - you're using a combination drug. These aren't just random pairs. They're carefully designed to work better together than alone. But here's the problem: not all versions of the same combo are created equal. Even if two pills have the same active ingredients, different doses, fillers, or release patterns can change how your body responds. This is where therapeutic equivalence comes in - and why getting it wrong can lead to real health risks.
What Therapeutic Equivalence Really Means
Therapeutic equivalence doesn’t mean two drugs are identical. It means they’re close enough that you can switch between them without losing effectiveness or safety. The U.S. FDA uses the Orange Book to rate drugs with letters: an ‘A’ rating means the generic is considered interchangeable with the brand. A ‘B’ rating means there’s uncertainty - maybe the absorption is different, or the formulation changes how the drug works in your body. For single drugs, this is straightforward. But when you combine two active ingredients - like simvastatin and ezetimibe in Vytorin - things get messy. Each component has its own dose-response curve. One might peak quickly; the other lingers. If the generic version changes the ratio of those components, even slightly, the whole effect shifts. A 2018 study found that 12% of patients on levothyroxine combos had unexpected changes in thyroid levels after switching generics - even though both versions had FDA’s ‘A’ rating.Dose Equivalency Isn’t Just Addition
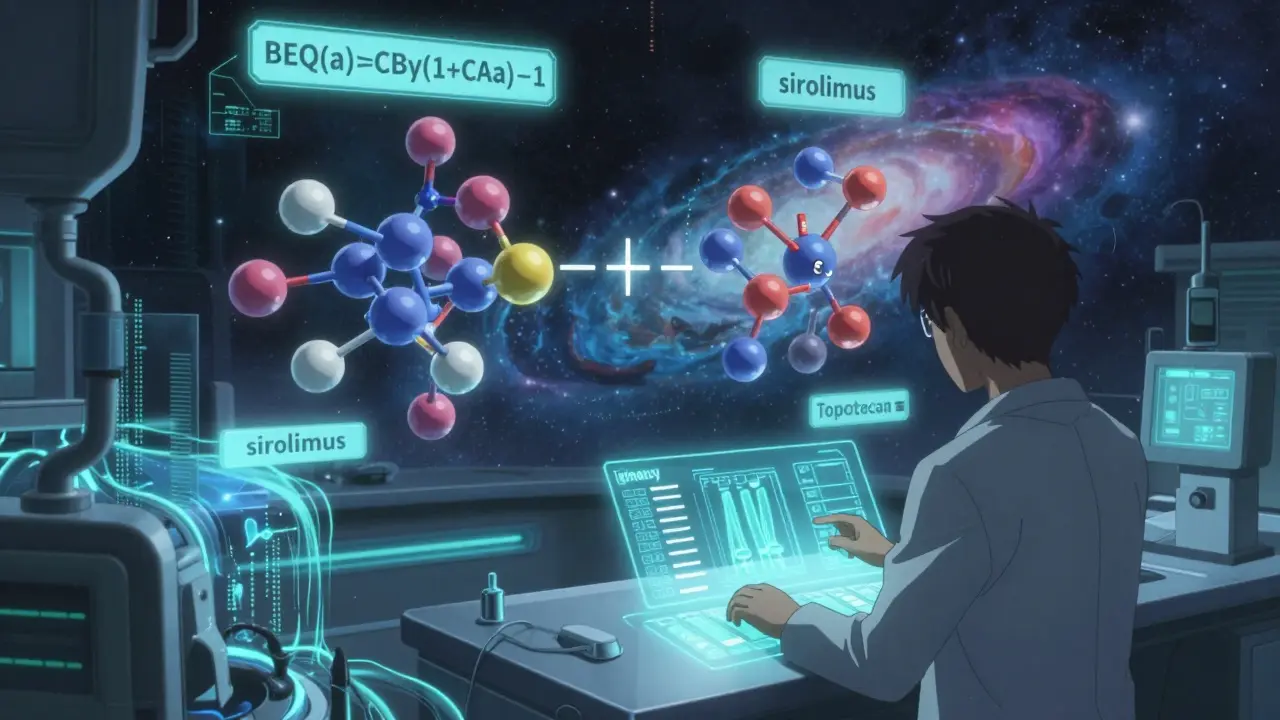
People assume that if Drug A and Drug B each reduce symptoms by 40%, combining them gives you 80%. That’s not how it works. Drugs interact. Sometimes they boost each other. Sometimes they cancel out. In a study of tramadol and acetaminophen, researchers found the combo worked better than the sum of its parts - a phenomenon called synergy. But if you swap one brand’s version for another, even with the same labeled doses, you might lose that synergy. The math behind this isn’t simple. Researchers use formulas like beq(a)=CBγ(1+CAa)−1 to calculate dose equivalents when two drugs have different maximum effects. For example, sirolimus reduces vascular growth by 69.8%, while topotecan hits 88.9%. To match their combined effect, you can’t just use equal doses. You need to adjust based on how potent each one is. This level of precision isn’t something pharmacists can eyeball. It requires detailed data - and most generics don’t publish it.Why NTI Drugs in Combinations Are Risky
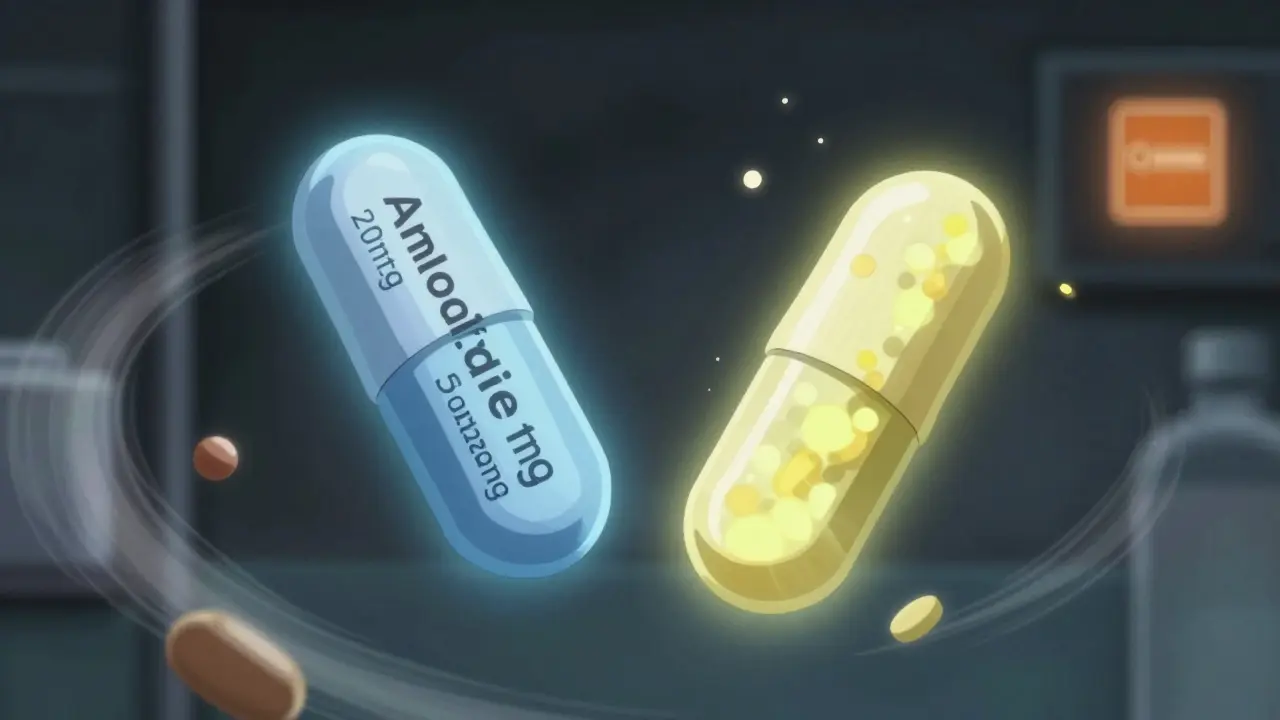
Narrow therapeutic index (NTI) drugs are the most dangerous when it comes to substitution. These are medications where the difference between a helpful dose and a toxic one is tiny. Think warfarin, lithium, phenytoin, or levothyroxine. When these are combined with another drug - say, levothyroxine with a statin - even a 5% change in absorption can trigger side effects. The FDA requires stricter bioequivalence standards for NTI drugs: 90-111% instead of the usual 80-125%. But that still leaves room for error. A 2022 FDA report found 247 adverse events linked to dose conversion errors in combination products. Nearly 40% involved cardiovascular combos. One patient switched from brand to generic amlodipine/benazepril and ended up in the ER with dangerously low blood pressure. The generic used a different filler that slowed absorption. The total dose was the same - but the timing wasn’t.
What Makes Two Combos Not Equivalent - Even If They Have the Same Name
Two pills can both be labeled “amlodipine 5mg / benazepril 20mg” and still behave differently. Why? Inactive ingredients. The coating, the filler, the disintegrant - these don’t show up on the label, but they affect how fast the drug gets into your bloodstream. Take rivaroxaban, an anticoagulant. There are seven generic versions, all with ‘A’ ratings. But three use croscarmellose sodium as a disintegrant. Four use sodium starch glycolate. In a combo with aspirin or clopidogrel, that small difference can delay absorption enough to increase clot risk. Pharmacists often don’t know which filler is in which batch unless they dig into the manufacturer’s documentation. Even more confusing: some combo products are approved under different FDA pathways. A traditional generic (ANDA) gets an ‘A’ rating. But a 505(b)(2) application - which tweaks the original formula - might get an ‘A’ or ‘B’ depending on whether the changes affect bioequivalence. You can’t tell just by looking at the bottle.How to Manage Combination Drugs Safely
If you’re prescribing or dispensing combination drugs, here’s what works:- Check the Orange Book - Don’t assume two combos are interchangeable. Look up the exact brand and generic names with their TE codes. If it’s ‘B’, don’t substitute.
- Stick to one manufacturer - Once a patient is stable on a specific generic version, keep them on it. Switching between generics - even with the same TE code - can cause instability.
- Use barcode scanning - Many hospitals now scan every combo drug at the point of dispensing. This links the exact product to the patient’s record and flags substitutions.
- Monitor for 72 hours after switching - Especially with NTI combos. Check blood pressure, INR, thyroid levels, or drug levels if possible.
- Document the brand and manufacturer - Not just the name. Write down the manufacturer. That’s the only way to trace problems later.
Real-World Mistakes and Fixes
A pharmacist in Ohio reported three dose errors in six months from switching between different amlodipine/benazepril generics. One patient got 10mg/40mg instead of 5mg/20mg because the pill looked similar and the system didn’t flag the difference. The fix? The pharmacy started using color-coded bins for each strength and required double-checking by two staff members. Another case: a nurse practitioner switched a patient from brand-name Vytorin to a generic. LDL cholesterol went up 15%. The patient had no symptoms - until a routine blood test. The generic used a different coating that reduced absorption of ezetimibe by 18%. The doctor had to switch back. Now, she only prescribes the brand for patients with stubborn high cholesterol. On the flip side, some systems have saved millions. A hospital in California implemented a therapeutic interchange program for proton pump inhibitor combos. They switched 80% of patients to a lower-cost generic with the same TE code. No adverse events. $1.2 million saved in one year.What’s Coming Next
The FDA is working on new tools to predict when a combo might not be equivalent. They’re using machine learning to analyze formulation data - filler types, particle size, coating thickness - and flag potential risks before the drug even hits shelves. Early tests are 89% accurate. In the future, we might see ‘A*’ ratings for combinations that prove equivalent across multiple strengths. And someday, pharmacogenomics could play a role. If you metabolize drugs slowly, your ideal combo dose might be different than someone else’s. By 2030, the NIH predicts 30% of therapeutic equivalence decisions will include genetic data. For now, the rule is simple: don’t treat combination drugs like single agents. Even if they have the same name, same dose, and same ‘A’ rating - they’re not always the same.Can I switch between different generic combination drugs safely?
Only if they have the same TE code (‘A’ rating) and you’ve confirmed the manufacturer hasn’t changed. Even then, it’s risky with NTI drugs or complex combos. Always check the Orange Book and monitor the patient for 72 hours after switching. When in doubt, stick with the original version.
Why do some generic combination drugs cost less but don’t work as well?
They’re not necessarily less effective - but they can be less predictable. Different inactive ingredients (fillers, coatings) can change how fast the drug is absorbed. For simple drugs, that’s usually fine. For combos, especially with NTI components, even small changes can throw off the balance. A 2022 study showed 18% of generic statin combos had slower absorption than the brand, leading to higher LDL levels in some patients.
Are brand-name combination drugs better than generics?
Not inherently. Most generics work just as well. But with combination products, brand-name versions often have more consistent formulations because they were the original. Generics must match the brand - but if the brand itself has variable absorption, the generic will too. The key is consistency: once you find a generic that works, stick with it.
What should I do if my blood pressure or cholesterol changes after switching to a generic combo?
Don’t assume it’s your lifestyle. Go back to your doctor or pharmacist. Request the exact name and manufacturer of the new generic. Check the Orange Book for its TE code. If it’s an ‘A’ rating, ask if the formulation changed. You may need to switch back or get a blood test to check drug levels. Many patients are told it’s “just a coincidence” - but it’s often the drug.
How can I tell if my pharmacy is substituting my combo drug without telling me?
Check the label. The manufacturer name and lot number are required. If they’re different from your last fill, a substitution happened. You have the right to refuse a generic substitution - even if it’s ‘A’ rated. Ask your pharmacist: “Is this the same manufacturer as before?” and “Can I get the brand if needed?”


evelyn wellding
January 17, 2026 AT 17:48Corey Sawchuk
January 19, 2026 AT 09:24Rob Deneke
January 21, 2026 AT 05:40kanchan tiwari
January 22, 2026 AT 06:28Bobbi-Marie Nova
January 23, 2026 AT 11:16Allen Davidson
January 24, 2026 AT 14:28Samyak Shertok
January 25, 2026 AT 21:39Stephen Tulloch
January 26, 2026 AT 00:04Joie Cregin
January 26, 2026 AT 03:05Melodie Lesesne
January 26, 2026 AT 08:48